When mathematical and computational models join forces with mechanobiology. This is a great statement to start this interview. Guillermo Vilanova is a PostDoc working together with Marino Arroyo at UPC. His aim within the Mechano·Control project is to develop a mathematical and computational framework to study the tightly coupled evolution of cancerous organoids and collagen matrix from a mechanical perspective. Let’s dive into the mathematical world and how it is connected with mechanobiology.
One of the aims of the Mechano·Control project is to achieve the mechanical control of the biological function with the aim to abrogate breast tumour progression. To meet this end, it is important to unravel the role of mechanical forces and cell mechanosensation in breast cancer invasion. To do that, Guillermo and the team at UPC are developing a mathematical and computational framework towards the understanding of the physical principles that drive this invasion.
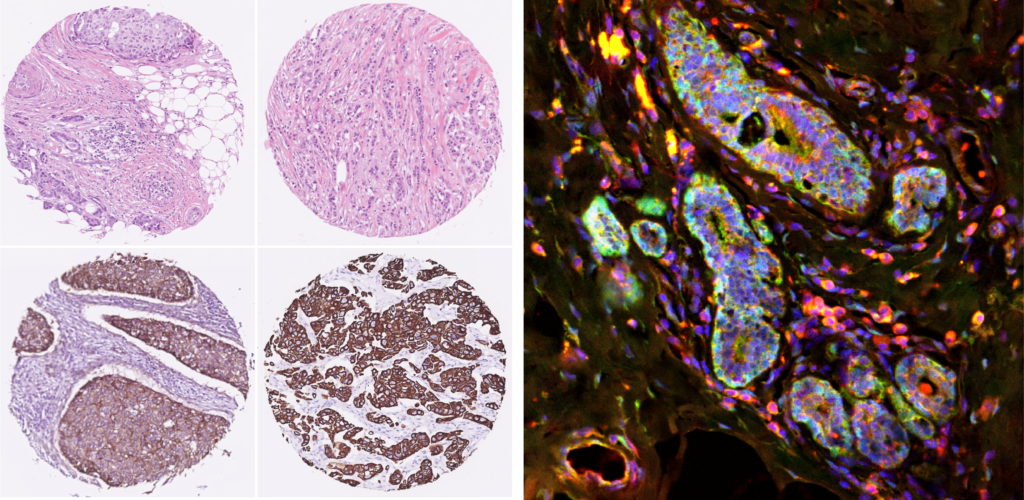
In ductal carcinoma, cancerous cells aggregate to form three-dimensional tumors in the ducts of the breast and may show a highly invasive behavior under certain conditions. Until now, the specific conditions under which ductal carcinoma becomes invasive has been elusive. The role of the extracellular matrix is being broadly studied and it has been demonstrated that it plays a crucial role in enhancing cell invasion towards other parts of the body.
Thanks to their collaborators’ latest experiments, they have proposed a mechanism for cancer invasion that views this phenomenon as a spontaneous pattern formation, and hence does not require that, by an unknown process, some specific cells change their nature and become leaders of invasion. Instead, the invading tumor self-organizes through a feedback between cellular mechanosensing and matrix mechanics. For this reason, Guillermo is currently working in developing more accurate models and simulations of this process to understand the mechanical basis of cancer invasion.
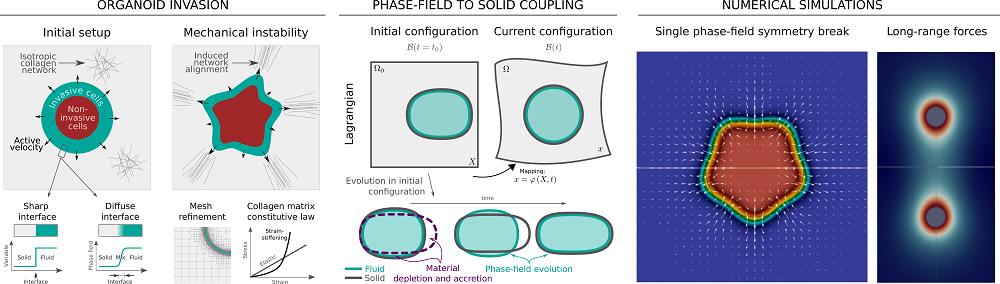
To this end, he is developing a mathematical model very similar to those used in the computation of forces in inert systems, like the models used to compute the forces that a pillar of a bridge needs to support. In biology, however, the challenge is that we deal with live and active systems, rather than inert. For instance, breast cancer cells can “sense” mechanical forces that are transmitted through the extracellular matrix and actively respond to them by changing their migration direction.
“Our mathematical model extends the classical equations for inert materials to incorporate these active mechanosensitive responses, the nonlinear behavior of the collagen-rich extracellular matrices and their complex coupling.”
Explains Guillermo Vilanova.
This mathematical model has two purposes: rationalize the observations made by their collaborators in their experiments and use this model as a predictive tool.
And how do they do that? On the one hand, by solving the equations of the mathematical theory in supercomputers to create simplified virtual replicas of the experimental setups. Then, by altering the conditions of these in silico experiments, they can understand what the key ingredients of the mathematical model are and thus, the driving forces of breast cancer invasion.
On the other hand, to use this mathematical framework as a predictive tool, thanks to the supercomputers, the researchers can create virtual playgrounds to test hypothesis and to unravel new mechanisms that can then be tested experimentally.
And which will be the expected results of these objectives? Again, we have two ambitious goals: address the importance of the relative positioning of cells within the tumor and unravel how cell mechanosensitivity and extracellular matrix mechanics interact to determine whether a tumor becomes invasive or not.
“The answer to these questions may deepen the knowledge we have on the role of mechanics in cancer invasion and may serve as a tool to ultimately prevent the invasion of breast cancer by altering the mechanical pathways.”
Adds Guillermo Vilanova
In order to understand and control how cells transmit and detect mechanical forces, it is important to take a look at every scale and use all the tools available. According to Guillermo, it is very exciting to be a part of this multidisciplinary and ambitious project that tries to answer such interesting and challenging questions. Although each member has specific expertise in different scientific fields and scales, we are working hand by hand in the same direction, so that by joining all the pieces we may finally solve the full puzzle. “It is extremely motivating to collaborate with people with different backgrounds that complement each other,” adds Guillermo.