In biomedical research, it is important to mimic the conditions that are found in our body. Traditionally, scientists have used 2D in vitro models where they grew cells on flat surfaces. However, as it can obviously be observed, the cells in our body are in three-dimension. So, wouldn’t it be amazing to create 3D models to study diseases, such as breast cancer?

Antoine Khalil is a senior Post-Doc at the UMC Utrecht in the Netherlands, in Johan de Rooij’s group. Their first and very important role at the Mechano·Control consortium was to establish the 3D in vitro models that recapitulate the characteristics of breast cancer in patients. They have used pieces of tumors (organoids) and grew them in 3D extracellular matrix (ECM). The ECM is a complex network of proteins that surround the cells in our body and provide important signals that control cell behavior. Antoine and his colleagues have characterized these cancer organoids and are trying to understand the nature of the extracellular matrix (ECM) that leads to cancer invasion and metastasis.
Working with organoids and 3D ECM makes in vitro studies more reliable, because they retain several aspects of tumor characteristics, and because most of the invasion that takes place in cancer patients, occurs in a 3D environment. The researchers can manipulate these organoids with the aim to stop the ability of cancer cells to become invasive and therefore, this will provide therapeutic opportunities to target the spread of the tumor cells throughout the body.
“We use a new type of model system for our research that is called organoid culture. Organoids are made from biopsy materials that are isolated from breast tumors in human patients or mouse models. In this way, we can grow a piece of tumor in a controlled environment and study the effects of specific ECM conditions on the induction of tumor cell invasion”, explains Antoine.
Invasion and spread of breast cancer into the surrounding breast tissue often happen in groups of cells (collective invasion). The invasion of the cancer cell groups is driven and guided by few specialized cancer cells called the leader cells. Antoine and his colleagues have identified and fluorescently-tagged molecules that are exclusively expressed in those leader cells. They also found that several of these molecules are activated by changes in the ECM stiffness and tension. Thanks to that, several of these models and markers are being used by the other members and institutions of the Mechano·Control project.
Antoine and his colleagues aim to unravel the molecules that are most important in the transition to an invasive leader cell state and suggest methods to interfere with this process to prevent breast cancer invasion and metastasis.
The “life-time” of a tumor goes through different stages: In the first stage, the tumor grows in an uncontrolled fashion. In this stage the tumor is still confined and the cancer cells remain at their site of origin. At a later stage, some cancer cells within the tumor undergo a transformation that makes them motile and invasive. When this happens, tumor cells can spread to different regions in the body (including lungs, liver and brain) to form secondary tumors (metastases). That is why understanding how invasion is regulated will allow blocking it and this is very important to prevent and reduce metastasis.
It is now very well known that stiffer extracellular matrices surrounding breast tumors strongly associates with breast cancer invasion. “Our major task in the Mechano·control project is to unravel the molecular machinery in breast cancer cells that is essential for ECM sensing and the acquisition of invasive behavior”.
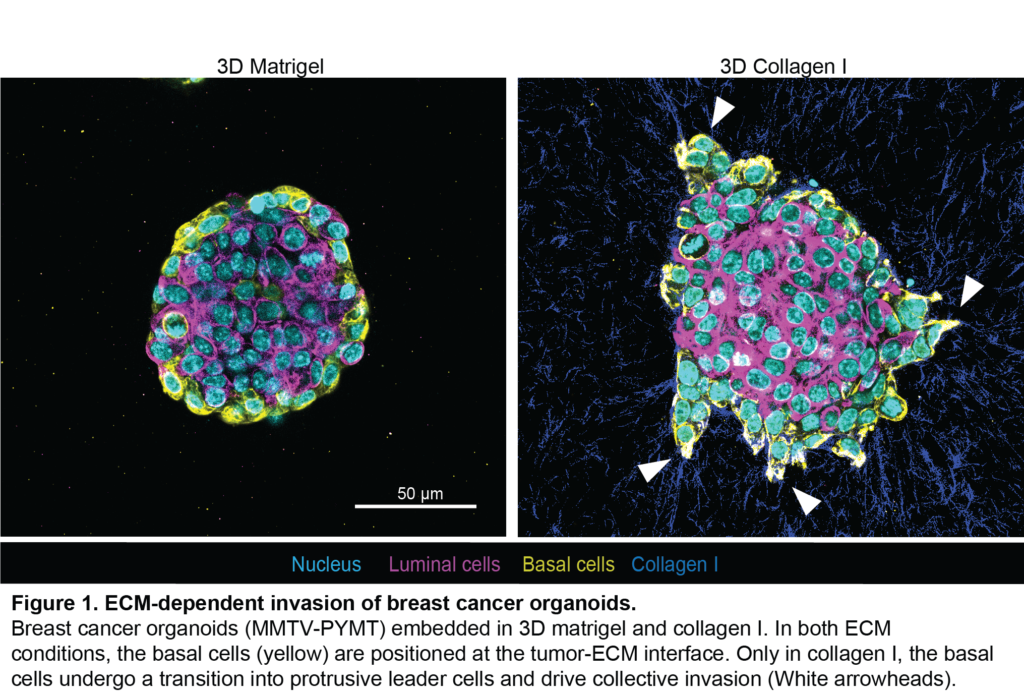
Until now, their data has established that only a pre-defined subset of breast tumor cells (the basal cells) (Figure 1, yellow) can become the leaders of invasion (Figure 1, white arrowheads). These leader cells drive the movement of groups of cancer cells. Antoine’s work shows that becoming a leader cell is triggered by changes in biochemical and mechanical properties of the ECM.
“We found that dynamic bidirectional interactions take place between the leader cells and the ECM. Changes in ECM biomechanics induce protrusive behavior in cancer cells, which causes further changes in the ECM that becomes permissive for directional cancer cell movement. We have identified a set of genes whose expression is activated in response to increase in ECM stiffness; many of those genes regulate ECM remodeling and cellular invasive behavior.”
Antoine Khalil, UMCU
This work and much more is what Antoine and his colleagues in de Rooij’s team have achieved within the Mechano·Control project, and we know that there is more exciting and promising research to come until the project ends. For Antoine being a part of the Mechano·Control community was a great opportunity for his work because this project brings together various research fields at very different scales, “Throughout our meetings, conferences and communications we have established strong connections with the partners and learned so much from experts in the cell-ECM interaction field, at the computational, nanoscale and molecular and cellular levels” tells Antoine.



