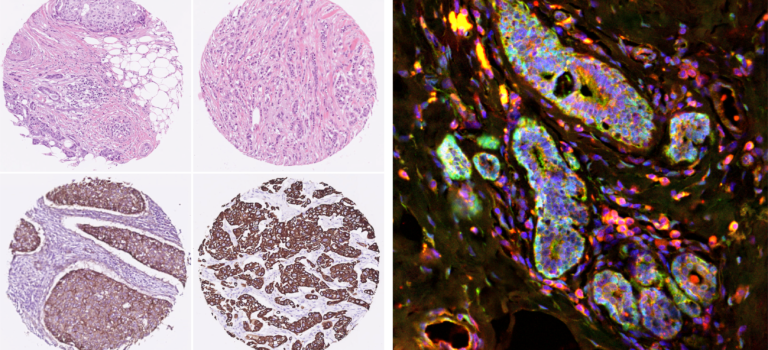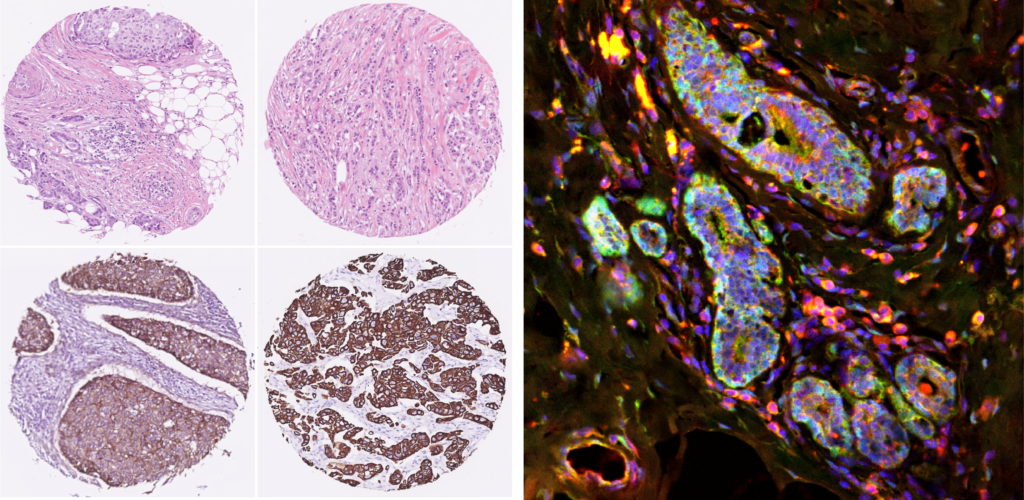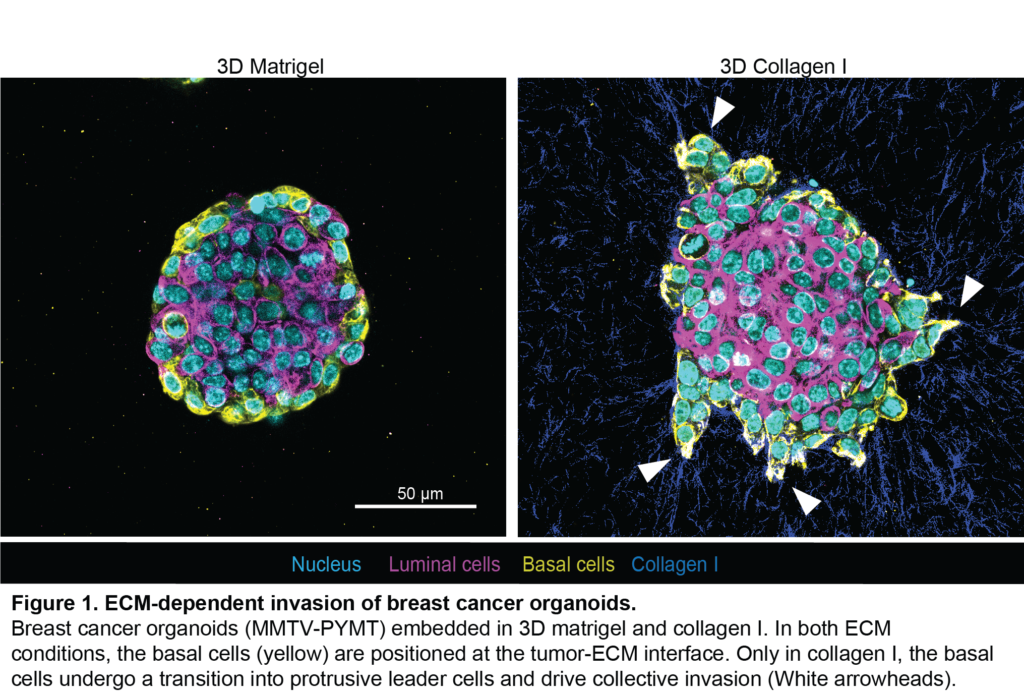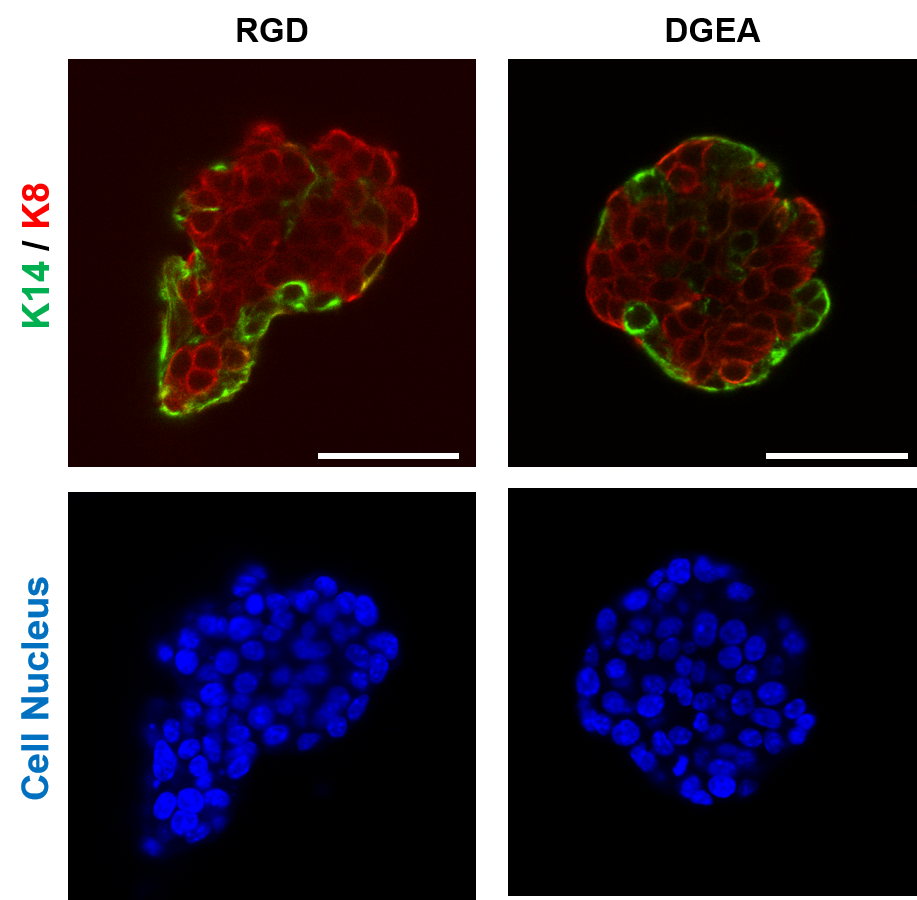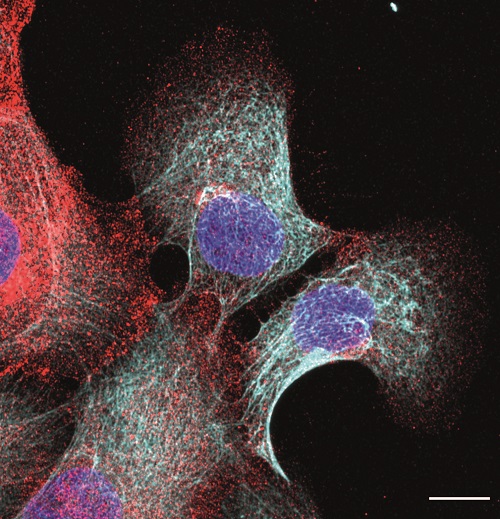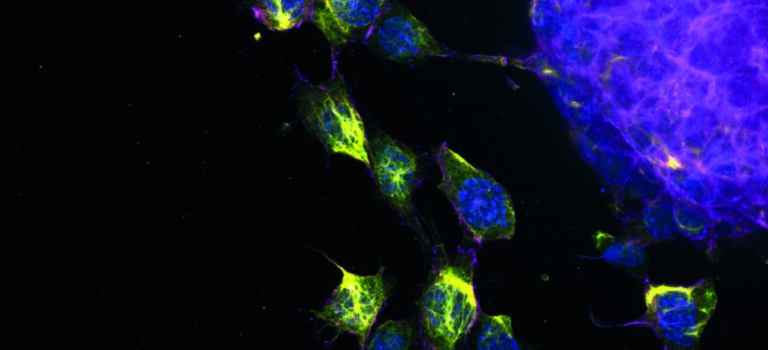
Breast cancer researchers at UMC Utrecht have discovered that a certain protein can predict whether a course of chemo will be effective for aggressive breast cancer. Women with breast cancer and a high level of FER protein have a greater chance of successful treatment with taxane chemotherapy. The results of this study were published in the renowned scientific journal Cell Reports.
Triple-negative breast cancer is an aggressive type of breast cancer. Women with triple-negative breast cancer often receive treatment with chemotherapy. The goal of the chemotherapy is to reduce the risk of metastasis (spread of the cancer) as far as possible, which increases the chances of survival.
The treatment with chemotherapy is not equally effective for all women. Many women also often suffer unpleasant side effects, such as fatigue, anemia, nausea, and hair loss. “It would be really helpful if we could predict whether a treatment with chemotherapy will be effective,” explains Patrick Derksen, professor of pre-clinical oncology at UMC Utrecht.
High FER protein, greater benefit of chemotherapy
His research group discovered that a certain protein could predict whether a course of chemo will be effective for triple-negative tumors. Women with breast cancer and high levels of the so-called “FER protein” will benefit more from treatment with chemotherapy.
Specifically, they will benefit from chemotherapy with taxanes, a type of medication that slows down cell division. “The FER protein basically recycles other proteins that the cancer cells need to be able to spread,” Derksen explains. “The taxanes slow down this process.”
Test that indicates whether chemotherapy will be beneficial
The researchers are now working on developing a test that demonstrates whether a triple-negative breast tumor has high or low levels of FER protein. High levels of FER equate to a greater probability of the taxane chemotherapy being effective.
Derksen: “We want to start using the test right from the moment of diagnosis, so that we can offer a more tailored treatment. The test is performed in the lab on collected tumor material. We do not need to ask the patients to do anything extra. We will conduct clinical trials on this test, to confirm our prediction and so be able to offer a more personalized and effective treatment.”
Further information
- Read the scientific article FER regulates endosomal recycling and is a predictor for adjuvant taxane benefit in breast cancer
- Profile page of Patrick Derksen
- Derksen lab website
Source: UMCUtrecht