Xavier Trepat, group leader at IBEC and PI of the Mechano·Control consortium together with Raimon Sunyer, Senior researcher in Trepat’s lab, have written a Primer in Current Biology magazine on “Durotaxis”, a cell migration mechanism that might have a role in several disease states that include the stiffening of tissues.
Embryo development, tumour progression or the immune response against pathogens requires cell migration. Cells are not static, they move and are able to direct their migration, normally guided by spatial gradients in a physicochemical property of the cell microenvironment, such as chemical concentration for example, but it is also guided by the stiffness of their extra-cellular matrix (ECM).
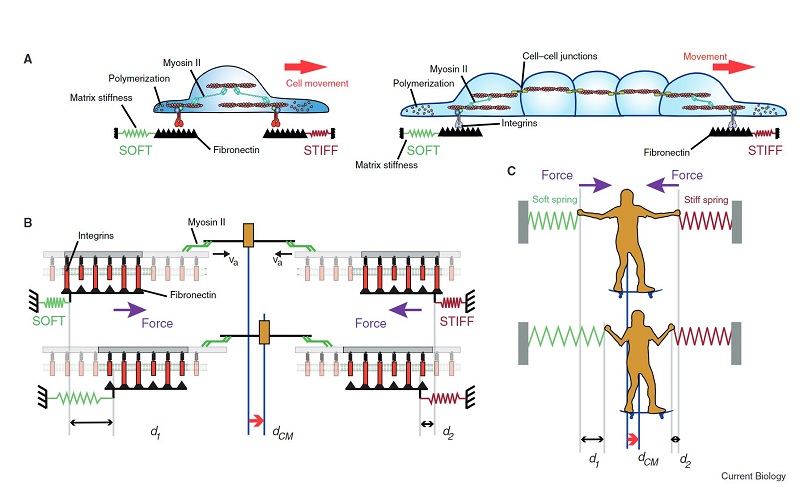
Durotaxis was first reported in 2000 and is the tendency of single cells to follow stiffness gradients. Since it was first described, several studies have been carried out, mostly in vitro, as in vivo remains poorly studied. As technological advances bring new tools to probe ECM stiffness in living tissues, new roles for durotaxis in vivo are likely to emerge.
Durotaxis is normally positive, towards stiff regions, but it has also been observed as negative, from stiff to soft, some examples of this phenomenon are explained in this review.
In this piece, written by Xavier Trepat and Raimon Sunyer, they give an overview on the methods used to study durotaxis both in vitro and in vivo, and on the state of art of the mechanisms of durotaxis, which remains incompletely understood.
The researchers also explain that some cell types do not display significant durotaxis when migrating in isolation, but they durotax efficiently as cohesive clusters. Multicellular clusters can behave as a giant supracell, increasing its sensitivity to mechanical gradients. However, collective durotaxis has not yet been demonstrated in vivo but it is believed that this phenomenon could guide collective migration in pathological processes involving local changes in tissue stiffness. For instance, solid tumours are widely known to be stiffer than the surrounding tissue, which may favour or prevent collective invasion.
In conclusion, in this review the researchers state that durotaxis is emerging as a robust mechanism to drive directed migration of single cells and clusters. Key components include cell-ECM adhesion through molecular clutches and long-range force transmission across the cytoskeleton and cell-cell junctions. It is expected that durotaxis might be the cause of many migratory movements in vivo that are currently unexplained.
Read the full article here: Raimon Sunyer; Xavier Trepat, Durotaxis. Current Biol. 2020, 30, R383-R387



