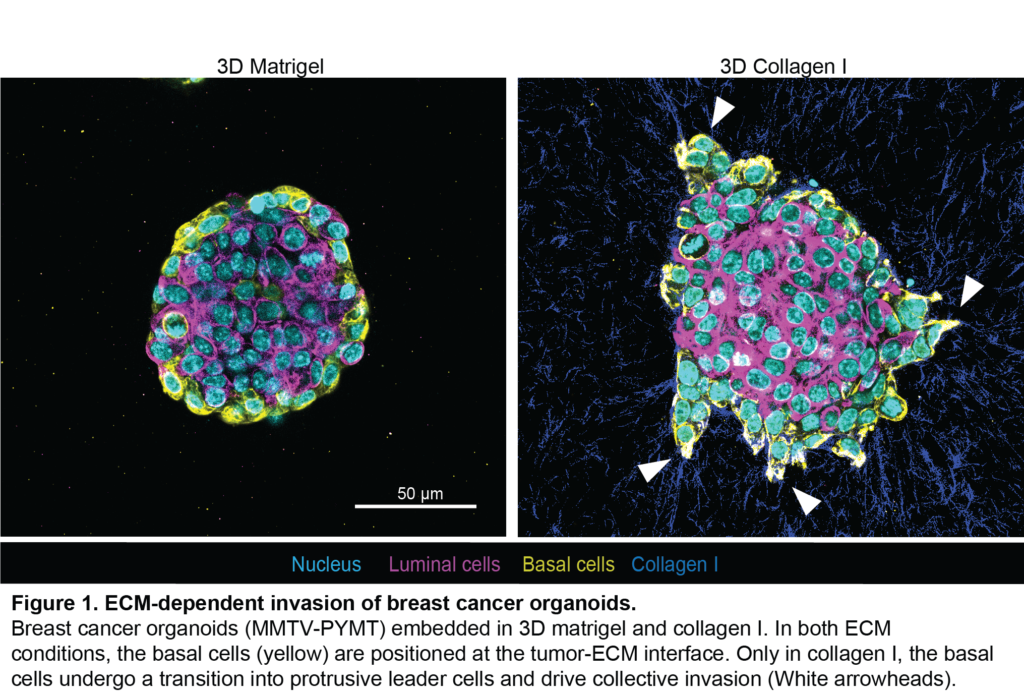
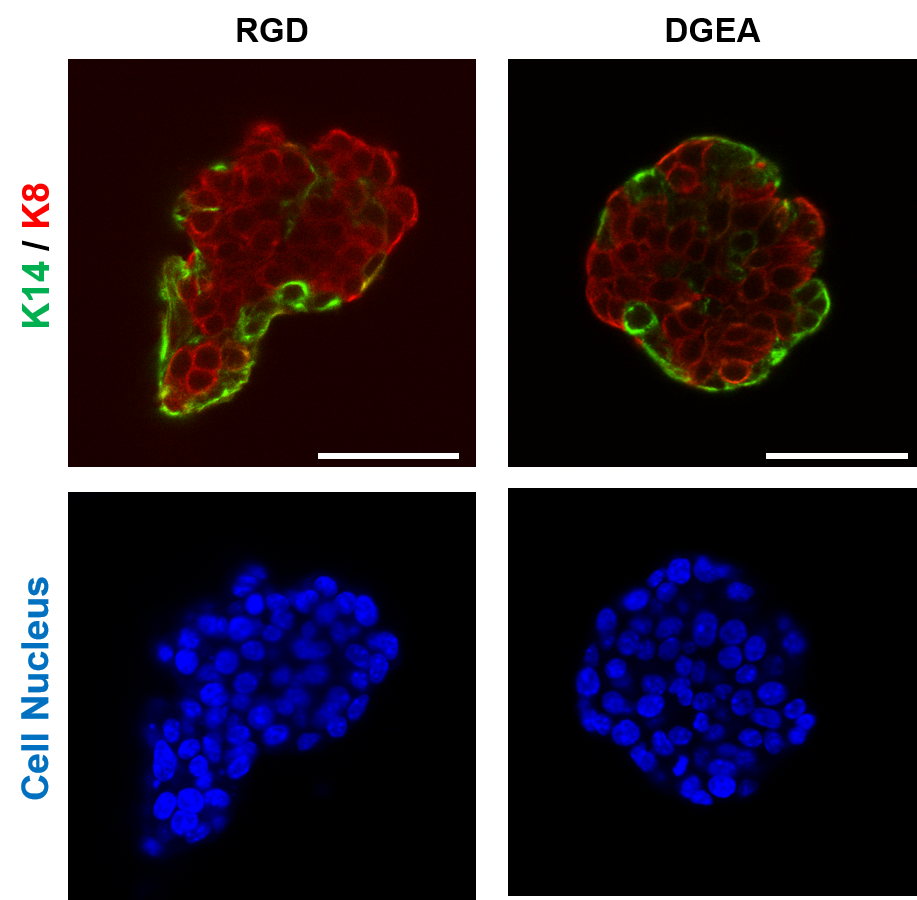
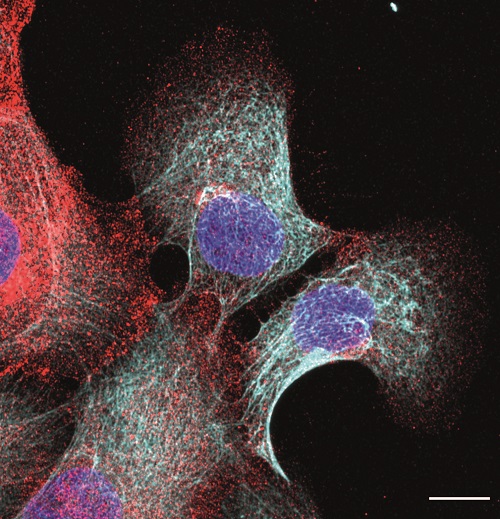
The MECHANO·CONTROL consortium, led by several research institutions across Europe, is launching the second edition of the “Mechanobiology of Cancer Summer School” which will take place between September 27th – October 1st 2022 at the Eco Resort in La Cerdanya. The aim of the summer school is to provide training on mechanobiology, and specifically its application to cancer. This school will include lectures as well as practical workshops in different techniques and disciplines, ranging from modelling to biomechanics to cancer biology.
The first edition was a great success, both in participation and scientific level, with more than 60 people from 13 different countries.

During this second edition, there will be scientific sessions in the morning, mixing 6 keynote speakers with 18 short talks selected from abstract submissions by junior scientists attending the school. In the afternoon, there will be 2-3-hour practical workshops, given by scientists from the MECHANO·CONTROL consortium. The course will also include leisure activities.
Attendance to the Summer School is open to all students, post-docs, and professionals interested, although priority will be given to junior scientists (up to post-doctoral stage).
You can visit the Mechanobiology of Cancer Summer School 2022 website, where you will find more information about the activities that will be held during the summer school, information on how to register, and the deadlines both for the registration and abstract submission.
The 6 confirmed speakers who will attend the summer school are:
- Dagmar Iber (ETH Zurich)
- Hans Van Oosterwyck (KU Leuven)
- Claudia Fischbach (Director of Cornell’s Physical Sciences Oncology)
- Giorgio Scita (IFOM – The Firc Institute Of Molecular Oncology)
- Madeleine J. Oudin (Tufts University)
- Maria Celeste Aragona (University of Copenhagen)
Also, all MECHANO·CONTROL consortium members will be attending the summer school and will be giving some of the whorkshops: Aránzazu del Campo (Leibniz-Institut für Neue Materialien, INM), Sergi Garcia-Manyes (King’s College London, KCL), Pere Roca-Cusachs and Xavier Trepat (Institute for Bioengineering of Catalonia, IBEC), Patrick Derksen, Antoine Khalil and Johan de Rooij (University Medical Center Utrecht, UMCU) and Marino Arroyo (Universitat Politècnica de Catalunya, UPC).
Preliminary list of workshop topics:
- Vertex modelling mechanics
- Single molecule mechanics
- Fundamentals of breast cancer biology
- Chemistry of tuneable gels
Don’t miss this great networking opportunity, where you will meet and interact with experts in the mechanobiology field and meet new people to collaborate with in the future!
About MECHANO·CONTROL
TheMECHANO·CONTROL project is focused on the mechanical control of biological function.Mechanical forces transmitted through specific molecular bonds drive biological function, and their understanding and control hold an uncharted potential in oncology, regenerative medicine and biomaterial design.
MECHANO·CONTROL proposes to address this challenge by building an interdisciplinary research community with the aim of understanding and controlling cellular mechanics from the molecular to the organism scale. At all stages and scales of the project, it will integrate experimental data with multi-scale computational modelling to establish the rules driving biological response to mechanics and adhesion. With this approach, it aims to explore novel therapeutic approaches beyond the current paradigm in breast cancer treatment. If the partners can understand cancer biomechanics from the single molecule to the whole organ scale, they’ll be able to control mechanical forces to restore healthy cell behaviour and inhibit tumour progression.
Beyond breast cancer, the general principles targeted by this technology will have high applicability in oncology, regenerative medicine, biomaterials and many other biological processes and diseases.
MECHANO·CONTROL is a project funded by the European Commission, within the Future and Emerging Technologies (FET) proactive program.