Samuel Pearson moved from Australia to Germany, where he works with research groups all over Europe with one common goal: to understand the factors influencing cancer progression. Samuel, Head of Applications in the “Dynamic Biomaterials” division of the Leibniz Institute for New Materials (INM), led by Prof. Aranzazu del Campo, is one of the Mechano·Control members. His work is focused on upscaling the production of polymer materials for 3D cell culture and bringing them towards commercialisation. Let’s take a closer look into his role within the consortium.
To understand how diseases like breast cancer develop, the response of breast cancer cells to different factors need to be studied one by one in isolation in 3D cultures. Samuel Pearson and his team produce synthetic hydrogels for 3D cell encapsulation with the possibility to adjust different parameters. These hydrogels mimic the properties of the natural breast cancer tissue in normal and disease conditions.
A particular interest of his group is the upscaling of the hydrogel precursors using affordable precursors and the design of stable, easy-to-handle compositions. This is the first step in technology transfer from the Mechano·Control project. With Mechano·Control partners, these materials are further developed into predictive breast cancer models.
Another crucial milestone for Samuel and his team is the processability of the hydrogels, which is related to the rate at which they are formed, i.e. the speed of the crosslinking reaction [1] during which cells are retained in a 3D matrix. The group has developed a patented crosslinking approach [2] based on thiol-methyl sulfone reactions which offer very convenient timescales for uniform encapsulation of cells. “In the future, we want to optimize our 3D cell encapsulation systems for automated high throughput methods that will enhance reproducibility and expand the applicability of our platform” explains Samuel. “The figure below shows how basal and luminal cells in breast cancer organoids organise in response to different adhesive ligands in the surrounding polymer network, which relies on consistent network properties” adds Samuel.
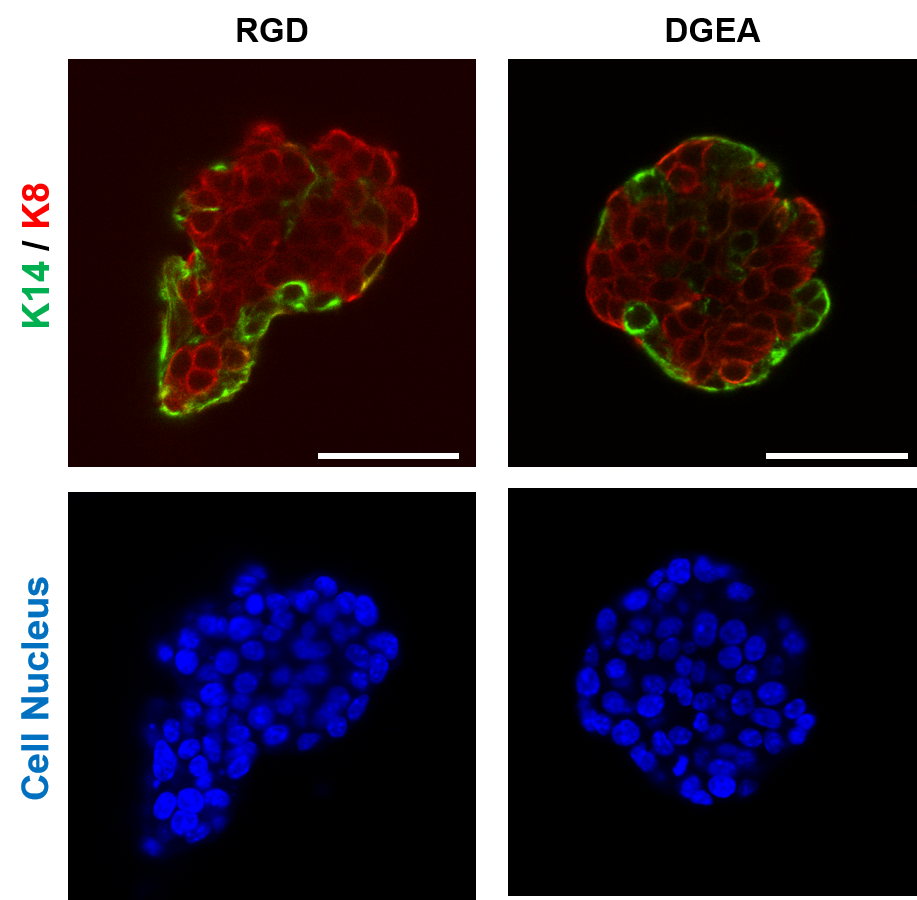
Figure 1: Invasive H7 breast cancer organoids were encapsulated in biofunctional hydrogels made from polymers developed at the Leibniz-INM. After 2 days of encapsulation, the effect of different bioactive ligands RGD and DGEA on polarization of the basal cells (stained with K14) and luminal cells (stained with K8) was explored. Scale bar = 50 um. Cell encapsulation and imaging were performed by Dr. Gulistan Kocer.
The materials developed by Samuel and his team play a crucial role in the Mechano·Control consortium. The high level of control and uniformity of the synthetic hydrogels allows to reduce the experimental variability in the biological studies, and this will help to uncover the individual factors that influence cancer progression. Moreover, this technology will not only be of great use for the consortium, but it also offers broad scientific and commercial potential for other 3D tissue culture models.
“We are optimising polymer structure and crosslinking chemistry to make hydrogels with very reliable properties. The focus right now is upscaling to allow high throughput studies and moving towards commercialisation”
Samuel Pearson
The Mechano·Control project brings together an interdisciplinary research community with the aim to understand and control how cells transmit and detect mechanical forces, from molecules to organs, and all the way up to the organism level. This requires developing and integrating disparate technologies, and each research group involved in the project is an expert in a very specific field and scale: “Each group has its own deep expertise, but because we are working towards a common goal everyone is really motivated to understand the contributions from the other partners, which deepens everyone’s knowledge and experience.”
[1] A crosslink is a bond that links one polymer chain to another, forming a network. Hydrogels are crosslinked networks of hydrophilic polymers that are swollen with water and widely used for 3D cell culture. The crosslink density is a key parameter that determines the mechanical properties of the hydrogel, which can in turn influence the behaviour of encapsulated cells. In the research described above, covalent crosslinks are formed using a patented thiol-methyl sulfone crosslinking reaction.
[2] del Campo, A.; Farrukh, A.; Paez, J. I. Novel Hydrogels. WO2021001203A1, 2021, Priority DE102019117997A 2019-07-03, Filed 2020-06-23, Published 2021-01-07.



